Leucine amino peptidase (LAP)
L-Type LAPFor In Vitro Diagnostic Use
Intended Use
L-Type LAP is an in vitro assay for the quantitative determination of the activity of Leucine aminopeptidase (LAP) in serum or plasma.
Method
L-leucyl-p-nitroanilide substrate method
Special Characteristics
Good stability after opening.
Calculate the measured K factor with 4-nitroaniline for setting the calibration coefficient of our product.
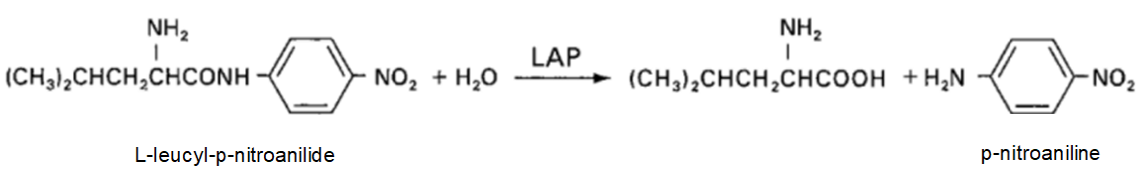
Principle of the Method
When a sample is allowed to act on L-leucyl-p-nitroanilide in phosphate buffer, p-nitroaniline is released by leucine aminopeptidase in the sample. The leucine aminopeptidase activity value in the sample is determined by measuring the production rate of this p-nitroaniline.
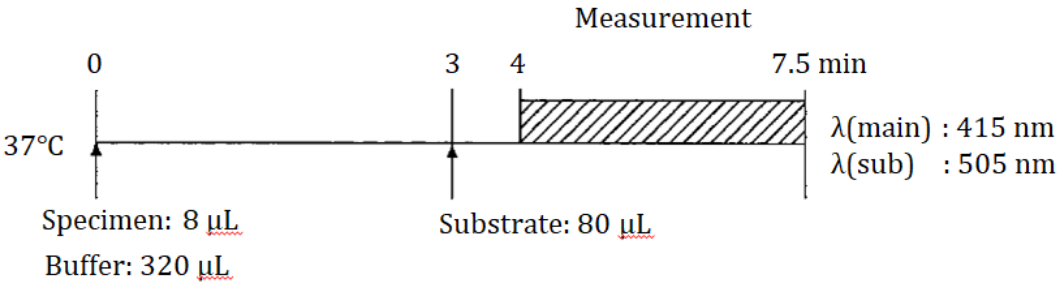
Standard Procedure
Ordering Information
| Product | Storage Condition | Shelf Life |
|---|---|---|
| L-Type LAP Buffer | 2-25℃ | 21 months |
| L-Type LAP Substrate | 2-10°C | 12 months |

